GGC’s Research Division actively studies a group of inherited diseases called the Congenital Disorders of Glycosylation or CDG. These rare diseases are caused by defects in enzymes and proteins responsible for adding sugars onto proteins and lipids, a process known as glycosylation. When proteins or lipids in the cell are abnormally glycosylated, their biological functions can become impaired. Patients with CDG have multisystem complications reflecting the central importance of glycosylation to the development and maintenance of most major organ systems in the human body. Once thought to be very rare, more and more CDG types that affect the different glycosylation pathways in the cell are being identified each year.
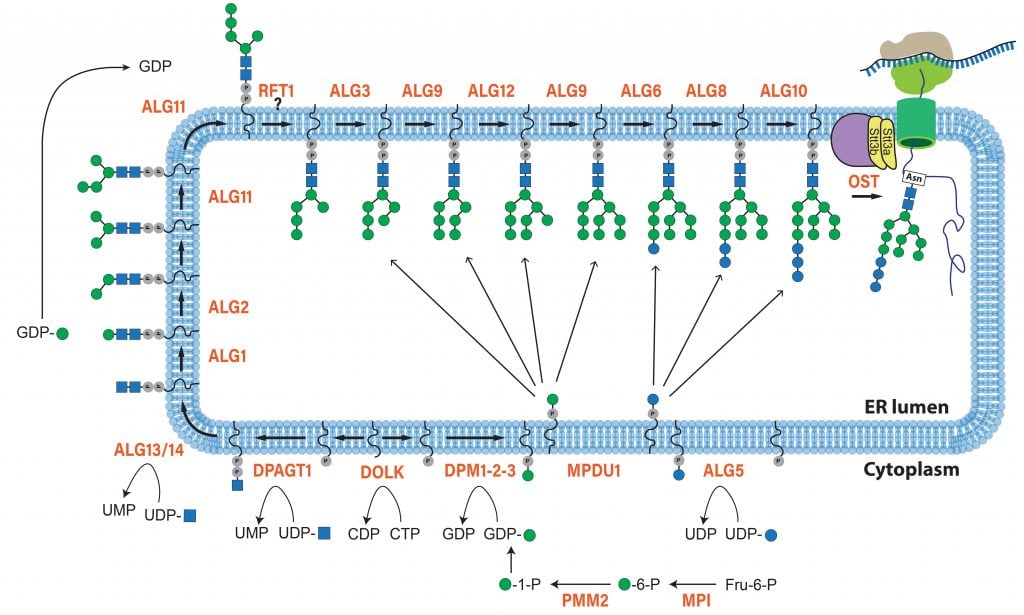
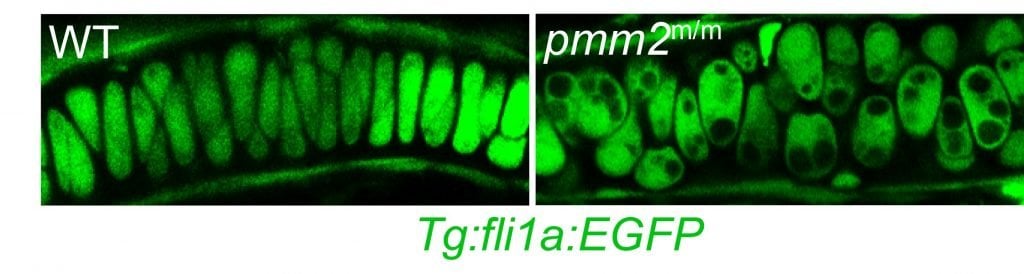
GGC researchers are studying CDG on numerous levels, with a major focus on understanding the pathogenesis of the most common CDG, PMM2-CDG. Their team is investigating how abnormal glycosylation causes the tissue-specific symptoms of this and other related CDG. They are using this information to explore novel treatments in partnership with multiple academic collaborators. The Division is taking advantage of novel zebrafish models to unravel the complex nature of CDG and identify the key proteins and pathways involved in the disease process. GGC researchers are working to identify the transcriptome and protein changes that are most sensitive to global defects in glycosylation. Much of this work is done in collaboration with other research groups around the country, including the following projects:
